Lupus Anticoagulants (LA) are a heterogeneous class of immunoglobulins which is one of the root causes of autoimmune diseases. The LA in combination with proteins such as β2-glycoprotein I (β2-GPI), prothrombin, or negatively charged phospholipids may prolong phospholipid-dependent coagulation tests.
An LA test is carried out to understand the cause of unexplained blood clot (thrombosis) in the vein or in an artery. The association of Deep Venous Thrombosis (DVT) and Anti-phospholipid Antibodies (APA) has been decisively established for patients with Systemic Lupus Erythematosus. The LA testing along with the solid phase of immunoassays, play a key role in determining the presence of APA in the plasma. Recurrent Pregnancy Loss (RPL) is associated with Anti-phospholipid Syndrome (APS), LA and Anti-cardiolipin Antibodies (ACA). Treatment on the basis of the diagnosis of the presence of LA may lead to successful pregnancies in women with an autoimmune disorder.
In cases of an unexplained prolonged Prothrombin Time Test (PTT), LA testing helps determine if it is due to a specific inhibitor, such as an antibody against a specific coagulation factor, or to a nonspecific inhibitor like the LA. The complications due to LA may lead to a stroke, recurrent spontaneous abortions, transient ischemic attacks and unexplained blood clot (thrombosis). In case of a hemorrhagic risk long-term anticoagulation, the accurate measurement of LA plays a crucial role in deciding whether and how long anticoagulation should be undertaken.
There are different diagnostic tests available for LA assays and different guidelines for pre-analytical, analytical and post analytical phases of LA testing. The guidelines have been proposed by -
- The International Society of Thrombosis and Haemostasis Scientific Standardization Committee (ISTH SSC)
- The British Committee for Standards in Haematology (BCSH)
- The Clinical and Laboratory Standards Institute (CLSI)
There are different LA assays done in clinical laboratories for the identification of LA. Some of them are the dilute Russell Viper Venom Test (dRVVT), Silica Clotting Time (SCT), Hexagonal Phase Phospholipid Neutralization (STACLOT-LA), Kaolin Clotting Time (KCT), dilute Prothrombin Time (dPT), Platelet Neutralization Procedure, etc.
In SCT, a colloidal silica suspension is used in the reagent to activate FXII in the intrinsic pathway. The patient sample is mixed with the reagent containing low concentration Phospholipid. This helps in increasing the sensitivity of the reagent to the presence of a LA, resulting in a prolonged clotting time. In the confirmatory assay, the patient sample is added to a second reagent containing an increased amount of Phospholipid. The excessive Phospholipid neutralizes the antibody by providing increased surface area for the intrinsic tenase and prothrombinase complexes, which reduces the clotting time. It is recommended to calculate both, an SCT screen ratio (patient screen result/mean of screen NR) and a SCT confirm ratio (patient confirm result/mean of confirm NR) and report the integrated result as a normalized ratio (screen ration/confirm ratio). In general, a ratio >1.16 is suggestive for the presence of a LA.
The Hexagonal Phase Neutralization is an integrated testing in which the patient’s plasma is mixed with the normal control plasma and incubated in the presence and absence of a Hexagonal Phase (II) phosphatidylethanolamine (HPE) phospholipid. An APTT testing assay is performed on both samples simultaneously using a LA sensitive reagent with low phospholipid concentration. If LA is present, it will result in a shorter clotting time compared to the sample incubated in the absence of HPE. Results are reported as the difference in clotting time between the tube with and without HPE. In general, a difference ≥ 8 is suggestive for the presence of a LA.
The dilute Russell Viper Venom Test (dRVVT) is recognized as the initial assay to be included when screening for a LA. This assay is based on the activation of Factor X at the beginning of the common pathway by an enzyme derived from Russell Viper (Daboia russelii) Venom. The FXa converts prothrombin to thrombin in the presence of phospholipids, calcium ions and FVa. In the next step, thrombin converts fibrinogen to fibrin. The dRVVT assay is not affected by deficiencies located in the extrinsic or intrinsic pathways of the coagulation cascade. The dRVVT usually consists of two components - the first component is a screening reagent and the second component is a confirmatory reagent. The first component contains a decreased amount of phospholipids in the reagent making it very sensitive to the presence of a LA (Figure 1). When the patient’s plasma containing an LA is mixed with the screening reagent, the clotting time is prolonged.
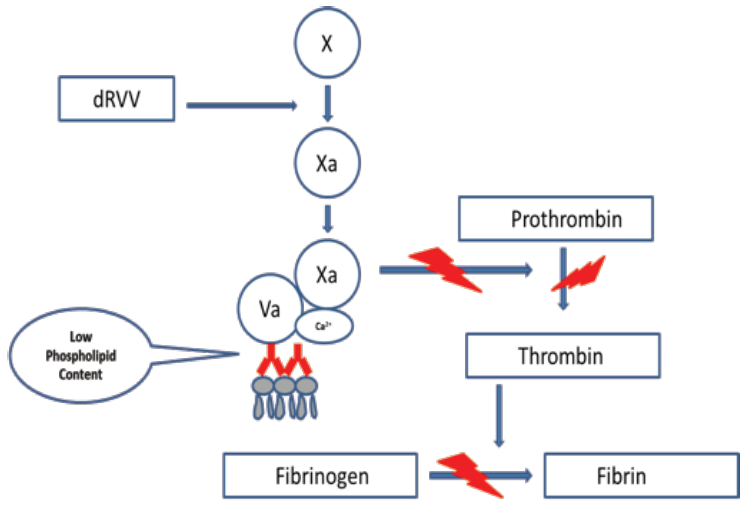 Figure 1: dRVVT screening assay. The screening assay requires a lupus sensitive reagent that contains a decreased amount of phospholipid. If a LA is present, it will interfere with the PC binding to the phospholipid surface of platelets.
Figure 1: dRVVT screening assay. The screening assay requires a lupus sensitive reagent that contains a decreased amount of phospholipid. If a LA is present, it will interfere with the PC binding to the phospholipid surface of platelets.
The second component has an increased amount of phospholipid added to the reagent that neutralizes the antibody by providing an increased surface area for binding of the prothrombinase complex (PC). Due to this clotting time reduces as compared to the screening assay (Figure 2).
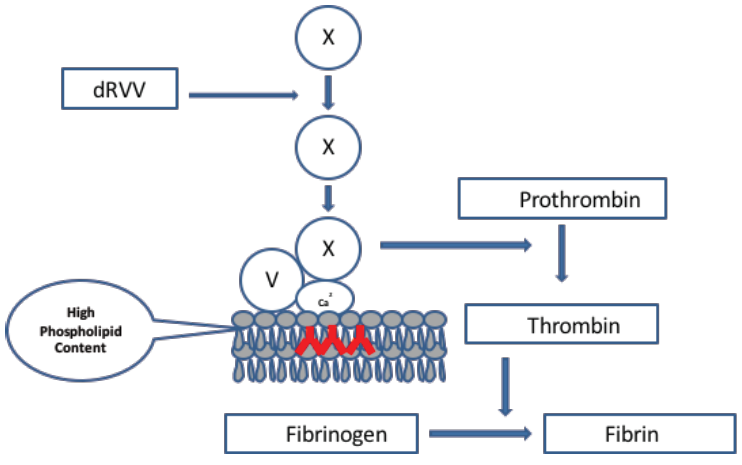 Figure 2: dRVVT confirm assay. The confirm assay requires a reagent that contains an increased concentration of phospholipid. If a LA is present, it will neutralize the antibody facilitating binding of the PC onto the platelet phospholipid surface
Figure 2: dRVVT confirm assay. The confirm assay requires a reagent that contains an increased concentration of phospholipid. If a LA is present, it will neutralize the antibody facilitating binding of the PC onto the platelet phospholipid surface
When the screening and the confirmatory reagents are used together, the assay is considered as an ‘integrated’ assay. As per the guidelines, it is recommended that laboratories calculate a dRVVT screen ratio (patient dRVVT screen result/mean of dRVVT screen normal range) and a dRVVT confirm ratio (patient dRVVT confirm/mean of dRVVT confirm normal range) and report the integrated result as a normalized ratio (dRVVT screen ratio/dRVVT confirm ratio). The ratio >1.2 is suggestive for the presence of LA.
At Transasia Bio-Medicals Pvt. Ltd., we follow dRVVT assay with two components; LA screening assay and LA confirmation assay. Russellʼs Viper venom directly activates Factor X to Factor Xa in the presence of phospholipid and calcium, leading to detectable clot formation in plasma.
Erba LA1 Screen kit is more sensitive for LA than the aPTT.
Erba LA1 Screen kit (Cat. No.: EHL00037) is intended to be used in conjunction with the Erba LA2 Confirm kit (Cat. No.: EHL00038).
If the clotting time of the patient’s samples with the Erba LA1 Screen procedure is greater than three standard deviations, above the mean of the normal range and are not corrected by mixing studies, a lupus anticoagulant may be present. Under these circumstances, samples should be re-tested using the Erba LA2 Confirm Reagent.
References:
- Armando Tripodi; Laboratory Testing for Lupus Anticoagulants: A Review of Issues Affecting Results; Clinical Chemistry 53:9 1629–1635 (2007)
- P.G. Degroot, B. Lutters, R.H.W.M. Derksen, T. Lisman, J.C.M. Meijers And F.R. Rosendaal; Lupus anticoagulants and the risk of a first episode of deep venous thrombosis; Journal of Thrombosis and Haemostasis, 3: 1993–1997
- JA Olaniyi, SA Olomu, OA Finomo; Lupus anticoagulant (LA) in pregnant women with history of recurrent fetal loss; Journal of Blood Medicine 2011:2 87–90
- Larry Smith; Laboratory Diagnosis of the Lupus Anticoagulant; Vol. 30, No. 1 Winter 2017 Clinical Laboratory Science